Determination of material

With electroplating and many other surface coating processes, the central question often arises in advance:
What material is the object to be coated made of?
To answer this important question, we provide you with a few test methods and tricks - after all, you want to know which metal you are dealing with so that you can coat it properly.
General test methods
1) Visual impression - what colour is my object?
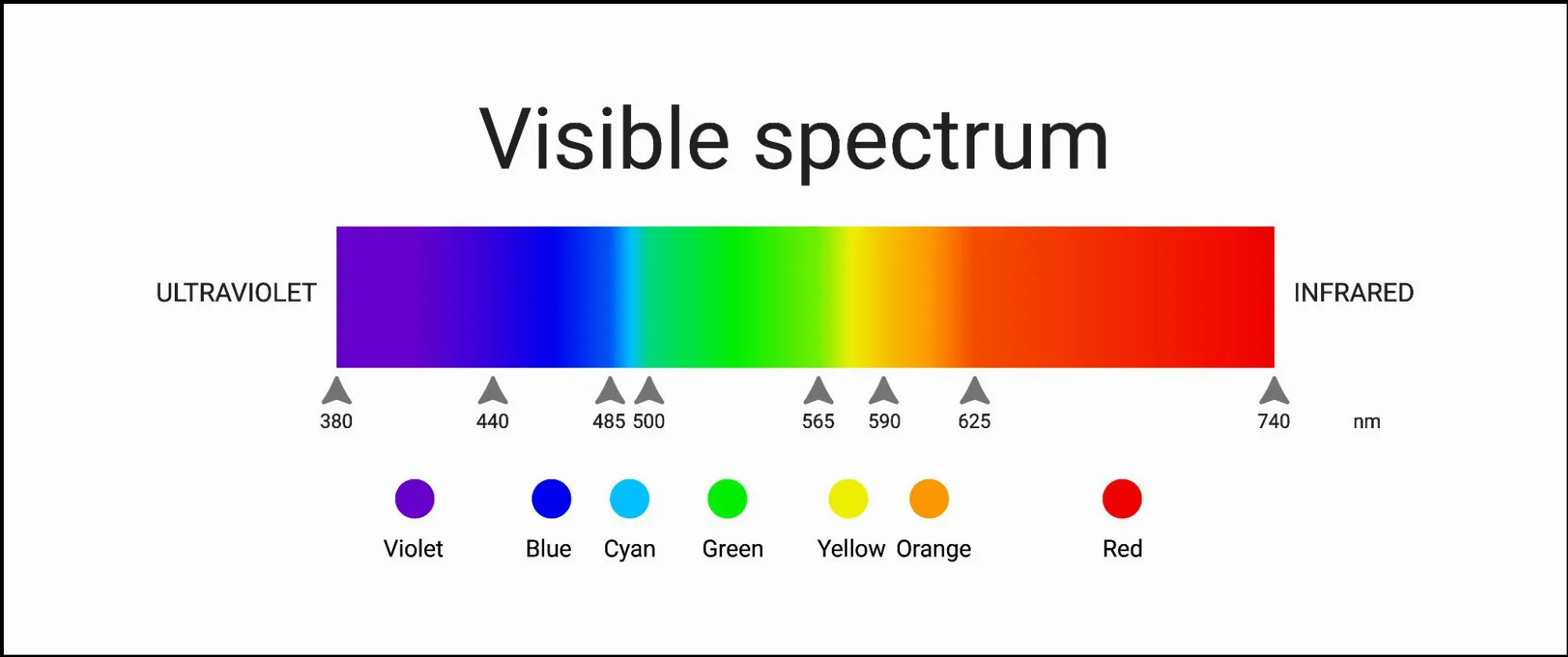
Most metals have a silver-grey colour in their pure form. However, there are also alloys that can produce different colour shades depending on their composition. The surface colour can also be changed by natural oxidation or patination.
In physical terms, the visible colour of a material is the combination of the reflected wavelengths of sunlight. For example, if a material mainly reflects green wavelengths and absorbs other wavelengths, it will appear green. Metals that appear grey reflect a large proportion of the visible light, pure copper reflects more in the red-orange colour range, and after oxidation mainly in the green wavelength range (patina). You can find reference points in the following table:
| Colour tone | Material |
|---|---|
| Grey, dull, matt | Tin, Tin casting |
| Blue-silver, glossy | Zinc |
| Fark grey, matt | Lead |
| Grey-silver, matt | Iron, low-alloyed steels |
| Grey-silver, glossy | Chrome, nickel, stainless steel |
| White-silver, glossy | Silver, platinum |
| Yellow-gold, glossy | Gold, brass |
| Golden-reddish, glossy | Gold alloy with copper content |
| Brown-reddish, glossy | Copper |
| Brownish-gold, glossy | Copper alloy, bronze, rose gold |
2) Magnet test - how strong is the magnetic attraction on my object?



The object can be tested for magnetism using a commercially available magnet. Magnetism is caused by magnetic fields generated by magnets, electric currents, or moving charged particles. These fields exert forces on other magnetic materials or charged particles. The following scenarios are possible with metals:
3) Hallmarking - does my item have a symbol or number embossed on it?

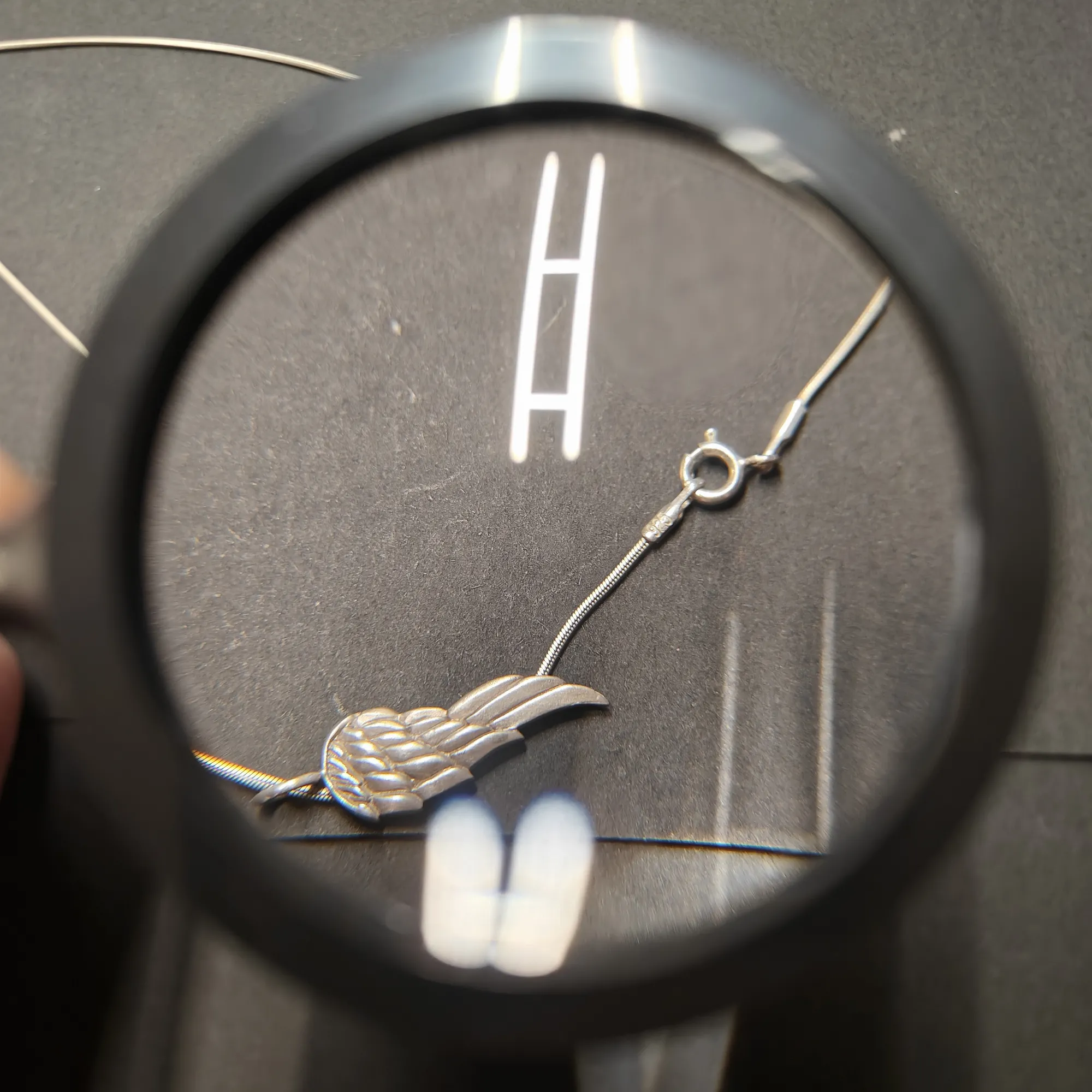

Jewellery made of gold, silver and platinum as well as silver cutlery and tin plates are often hallmarked. This means that a symbol or number is stamped into the item with a hallmark, which is used to indicate the degree of purity or the strength of the alloy.
4) Differences due to weight and density
Aluminium and aluminium alloys differ from heavy metals in their low specific weight. Aluminium has a low density of 2.7 grams per cubic centimetre. Iron, on the other hand, has a density of 7.8 grams per cubic centimetre.
5) Oxidation and oxidation colours

Rust formation in low-alloy steels and iron

Green patina or copper patina oxidation

Black colouring of pure silver
6) Plausibility - which metal do I probably have in front of me?
Certain objects are usually made of the same metal or similarly coated, here is a small selection:




Window handles:
Handles for everyday use are usually made of aluminium or aluminium alloys, which can be coloured green, blue, black or red by anodising or darkened by aluminium burnishing.


Fashion jewellery:
Fashion jewellery is often made of gold-plated plastic or gold-plated base metal. Before electroplating, a conductive intermediate layer is applied, e.g. copper conductive lacquer or palladium.
The metals at a glance

Aluminium

Copper/copper alloys:

Stainless steel

Iron/steel

Nickel
Nickel is a silver-white, corrosion-resistant metal. Nickel is magnetic and has weak oxidising properties. It is almost never found in its pure form. It is usually alloyed with iron and chromium (V2A steel). Nickel-plated surfaces are rare as they have an allergenic effect. Nickel coatings are usually gold-plated or chrome-plated, as nickel forms an ideal intermediate layer alongside palladium. Strict regulations apply to the sale of nickel and nickel solutions in Germany.
Occurrence: Alloys, e.g. stainless steels and inexpensive jewellery, (intermediate) coatings, nickel coins.

Silver

Chrome and chrome alloys

Platinum

Tin

Zinc
Disclaimer
The information provided here is a rough estimate and therefore without guarantee. We do not guarantee the accuracy and completeness of the above information. A binding material analysis cannot be made, at most an estimate of the benefits for electroplating.

.jpg)