New to electroplating? Valuable tips for implementing the first project

Material determination as preparation - what material do I have in front of me?
Choosing the right electrolyte for your electroplating project

- The object to be plated has to be free of dust and grease. The cleaner and smoother the surface BEFORE plating, the cleaner and better the result AFTER plating.

Avoid so-called carry-over effects, i.e. contamination of the electrolyte or similar, by rinsing with distilled or clean water after each step.

It is also essential to follow the safety instructions given in the application instructions. Safety goggles, mask and gloves are indispensable for any electroplating process.
Short version
- Determine the material of the object to be coated and check for compatibility.
- Select the correct electrolyte in the right size for the electroplating process.
- Carry out pre- and post-treatment. Wear protective clothing. Intermediate polishing if necessary.
Frequently asked questions
Wir haben dafür ein FAQ erstellt, um häufige Fragen schnell zu beantworten: Die Stabilität der Sperre ist von verschiedenen Faktoren abhängig, wie dem pH-Wert des Mauerwerks oder der Lichteinwirkung. Da diese Faktoren im Mauerwerk für die Funktionsweise von TOBOLIN günstig sind, kann davon ausgegangen werden, dass eine mit TOBOLIN eingebrachte Kapillarwassersperre bei korrekter Anwendung mehrere Jahrzehnte wirkt. Sollte die Sperre mit der Zeit schwächer werden, kann TOBOLIN an den entsprechenden Stellen einfach erneut eingebracht werden. https://www.tobolin.de/haeufig-gestellte.../wirkung-dauer
Aluminium and titanium are suitable for anodising. We recommend our products exclusively for aluminium.
Our products cover the majority of the most common aluminium alloys. However, the industry is constantly evolving. For an overview table of compositions suitable for anodising, please see our anodising guide.
In this case, the concentration of the colour solution is not high enough. We recommend 10g/L. Also possible: the alloy is not or only insufficiently suitable for anodising. You can find an overview table on this in our instructions for anodising.
When anodising, the surface area of the workpiece is calculated in dm² and multiplied by 1.5. This gives the necessary amperage for your project. As soon as you set this on your machine, the voltage regulates itself automatically.
No. Only the aluminium piece and titanium wire may be immersed in the solution with the anodising bath. The alligator clips must not come into contact with the bath.
This is most likely because the remnants of the bluing agent have not been rinsed off cleanly enough. This causes components of the bluing to crystallise on the surface and form a reddish-brown layer that looks like rust. Make sure that residues of the bluing liquid are completely removed at the beginning and end and that the surface is clean. Repeat the blackening process and wash off the bluing chemical carefully and completely at the end!
Our product range currently allows us to burnish the following materials: Iron, steel, zinc, aluminium and various non-ferrous metals. Check the product detail page for compatibility with the material you have.
Our bluing agents primarily create a black coloured surface that is not a real coating but inhibits the existing surface of steel, stainless steel or aluminium. Corrosion protection is only achieved if the blackened layer is oiled or painted at the end.
Tobostick-Verfahren wurde im Gegensatz zum Schrägbohrverfahren speziell dafür entwickelt, Hohlräume im Mauerwerk zu überbrücken. Darüber hinaus ermöglicht es die Erstellung einer waagrechten Horizontalsperre, was gerade bei hohen Mauerstärken Vorteile bietet. Die Injektionsflüssigkeit wird durch den Kapillareffekt vom Mauerwerk aufgenommen - die Flaschen werden durch diesen Effekt leer, obwohl sie sich unter dem Bohrloch befinden. Natürlich bieten wir aber auch das dir bekannte Schrägbohrverfahren an.
https://www.tobolin.de/anleitung-fuer-das-schraegbohrverfahren
https://www.tobolin.de/anleitung-fuer-das-tobostick-verfahren
The depletion of the electrolyte can be recognised by the slowing deposition. The colouring of the gold electrolyte Midas, for example, does not indicate that the electrolyte has been used up.
For gold and palladium: stainless steel or graphite. For copper (basic and acidic): Copper. For nickel electrolyte: nickel. For silver electrolyte: silver. For zinc electrolyte: zinc. For chrome electrolyte: not compatible with bath electroplating.
This varies from electrolyte to electrolyte. If the anodes are made of the same material as the salt in the electrolyte solution (e.g. copper, nickel, silver, zinc), the salt content remains constant because the anode dissolves slowly but steadily. However, the electrolyte is still contaminated at some point and brighteners (if present) are used up.
Certain electrolytes can be diluted. The gold electrolytes Flash and Midas can be diluted in a ratio of up to 1:1. However, the speed of deposition and the shine then decrease rapidly.
No, unfortunately that is not possible. Our chrome electrolyte only works in conjunction with the brush process (tampon electroplating).
The anodes should be at least as large as the surface of the object to be coated. The supply of 2 anodes (left and right) has been proven to give better results.
You have to multiply the current density recommended for the respective electrolyte by the surface area of the workpiece in dm² and then get the required current intensity. The voltage will readjust when the current is set accordingly.
If you want to coat from both sides at the same time, you should place an anode in the tank on each side and place the workpiece in the middle. The deposition is then more uniform than with the method with only one anode.
For diamonds/gemstones, carat is a measure of weight. Here, one carat corresponds exactly to 0.2 grammes. In the precious metal trade, on the other hand, the gold content of bars and coins is indicated as the fineness or fine gold content, usually in the form of an embossing. The CaratScreenPen measures the carat number of gold within seconds. Pure gold bars are stamped 999.9, also known as "four nine fine".
Below you will find an overview of the carat specifications for gold:
8 Karat:
333
Gold 33.3 %
9 Karat:
375
Gold 37.5 %
10 Karat:
417
Gold 41.7 %
14 Karat:
585
Gold 58.5 %
18 Karat:
750
Gold 75 %
20 Karat:
833
Gold 83.3 %
21 Karat:
875
Gold 87.5 %
22 Karat:
916,66
Gold 91.666 %
24 Karat:
999
Gold 99.9 %
Precious metals always offer investors a kind of security during crises. More common metals such as gold and silver can thus also be used as a medium of exchange, but more unknown precious metals are associated with a risk for precisely this reason. The respective prices are usually very dependent on the market and can fluctuate greatly depending on events. Safe investments are gold, silver, platinum and palladium.
Gold
Gold has the chemical symbol Au (Aurum). Although gold is also used in medicine, for example, it is traded less as a raw material and more as a pure investment metal.
Data on Gold 999:
Colour: yellow, gold
Density: 19.3 g/cm3
Conductivity: 45 MS/m
Silver
Silver has the chemical symbol Ag (Argentum). The precious metal is extracted from silver, copper and lead ores. Unlike gold, silver is traded on the stock exchange as a raw material, which is also needed in industry. Small investors can buy silver in the form of up to five kilo bars and coins.
Data on Silver 999:
Colour: shiny greyish, silver
Density: 10.5 g/cm3
Conductivity: 61 MS/m
Platinum
Platinum has the chemical symbol Pt. It is one of the most valuable precious metals of all, as it occurs in much smaller quantities than gold, for example. Due to its corrosion resistance, it is used in medicine as well as in industry. Platinum is traded in the form of bars and coins.
Data on Platinum 999.5:
Colour: greyish, silver
Density: 21.45 g/cm3
Conductivity: 9.1 MS/m
Palladium
Palladium has the chemical symbol Pd. It belongs to the platinum metals, which makes it valuable. Palladium occurs in combination with copper, nickel, gold and silver ores. It is mainly used in dental technology. It is also traded in the physical form of bars or coins and can be acquired by small investors as an investment.
Data on Palladium 999.5:
Colour: greyish, silver
Density: 11.99 g/cm3
Conductivity: 9.2 MS/m
Copper
Copper has the chemical symbol Cu. It belongs to the group of coinage metals. It is also very conductive and easy to process. It is mainly found in the automotive and mechanical engineering industries, but now also in renewable energies. For investors, it can be bought in the form of bars as well as coins.
Data on Copper 999:
Colour: reddish brown, metallic, copper-coloured
Density: 8.92 g/cm3
Conductivity: 58.1 MS/m
Rhodium
Rhodium has the chemical symbol Rh and belongs to the platinum metals. It occurs very rarely in nature, which is why it is extremely expensive. Consequently, its use is not common, but rhodium is found mainly in automotive catalytic converters. Investment rhodium is also available in the form of ingots.
Data on Rhodium 999:
Colour: silvery white, metallic
Density: 12.38 g/cm3
Conductivity: 18.5 MS/m
Iridium
Iridium has the chemical symbol Ir and is the second most dense element after osmium. It is one of the rarest, non-radioactive metals of all. Iridium is mainly used in mechanical engineering and medicine. The Urkilo, which is kept in Paris, and the Urmeter, which is also located there, are also made of an iridium alloy. The precious metal is very valuable and is traded on the stock exchange. Iridium bars and coins are also available for small investors.
Data on Iridium 999.5:
Colour: greyish, matt silver
Density: 22.56 g/cm3
Conductivity: approx. 19.7 MS/m
Ruthenium
Ruthenium has the chemical symbol Ru and belongs to the platinum metals. It is very rare and is only used in small quantities. Main applications of the metal are in the electronics industry. Some complexes of the metal are being researched for their effect as an anti-cancer agent. For investors, ruthenium can be purchased in ingot form.
Data on Ruthenium 999:
Colour: silvery white metallic
Density: 12.37 g/cm3
Conductivity: 14.1 MS/m
Memberships and certifications:
Official gold dealers usually have memberships such as with the "Berufsverband des Deutschen Münzenfachhandels e.V." or a "Good Delivery" certification from the "London Bullion Market Association". Members and certifications of this kind can be viewed publicly on the respective sites.
"Spot Price":
Dealers are tied to the current price of gold, the spot price and do not sell below value. If you find a particularly cheap offer on the Internet, you'd better watch out.
Service Tests:
Credible gold dealers can also be found in service tests. Test series by "Focus Money" or "Euro am Sonntag" regularly review various providers, which you can use as a guide.
Homepage and communication:
You can already unmask scammers on their homepage. Compare the prices of the offers with the current gold chart.
Personal contact with the dealer:
Reputable dealers always offer you personal contact as well and can be reached by phone at any time.
Product Description:
Here you can also immediately recognize bad counterfeits. Reputable gold dealers usually offer you high-resolution pictures of the products, while counterfeiters usually offer inferior ones to disguise obvious external differences. "Gold plated" does not mean solid gold, "fine gold plating" is nothing more than foreign metals covered with a thin layer of precious fine gold. You should also interpret expressions such as "replica", "medal", "replica" or even "counterfeit" as warnings.
The magnetic scale can be used for a wide range of objects. For example, bars weighing up to 250 g can be examined, as can common coin sizes up to 5 oz. For bars weighing more than this, we recommend additional test with our bar testing kit, as foreign metal inclusions behind gold layers thicker than 2.5 mm may not be detected by the magnetic balance. However, such thick coatings are still the exception, at least for the time being (a fact that may change with developments such as the magnetic balance).
Caution: The magnetic field of the scale could damage sensitive devices such as watches. Check this in advance and do not place objects of this type carelessly on the magnetic scale. Translated with DeepL.com (free version)
Under certain circumstances, a counterfeit can produce a positive, i.e. diamagnetic, result on the magnetic balance if the foreign core is hidden too deep inside and thus the paramagnetic "false core" is not detected by the diamagnetic layer. The maximum thickness of a gold layer through which measurements can be taken by the MagneticScreenScale is approximately 2.5 mm.
Likewise, a coating made of a strongly diamagnetic material that produces a strong positive value (e.g. bismuth) can, so to speak, compensate for the paramagnetic core lying further inside (which produces a negative value) and then falsify the value in the direction of "+". However, such forgeries would be difficult to produce and would require a relatively thick layer, e.g. bismuth, and would therefore be conspicuous in the weight or density test.
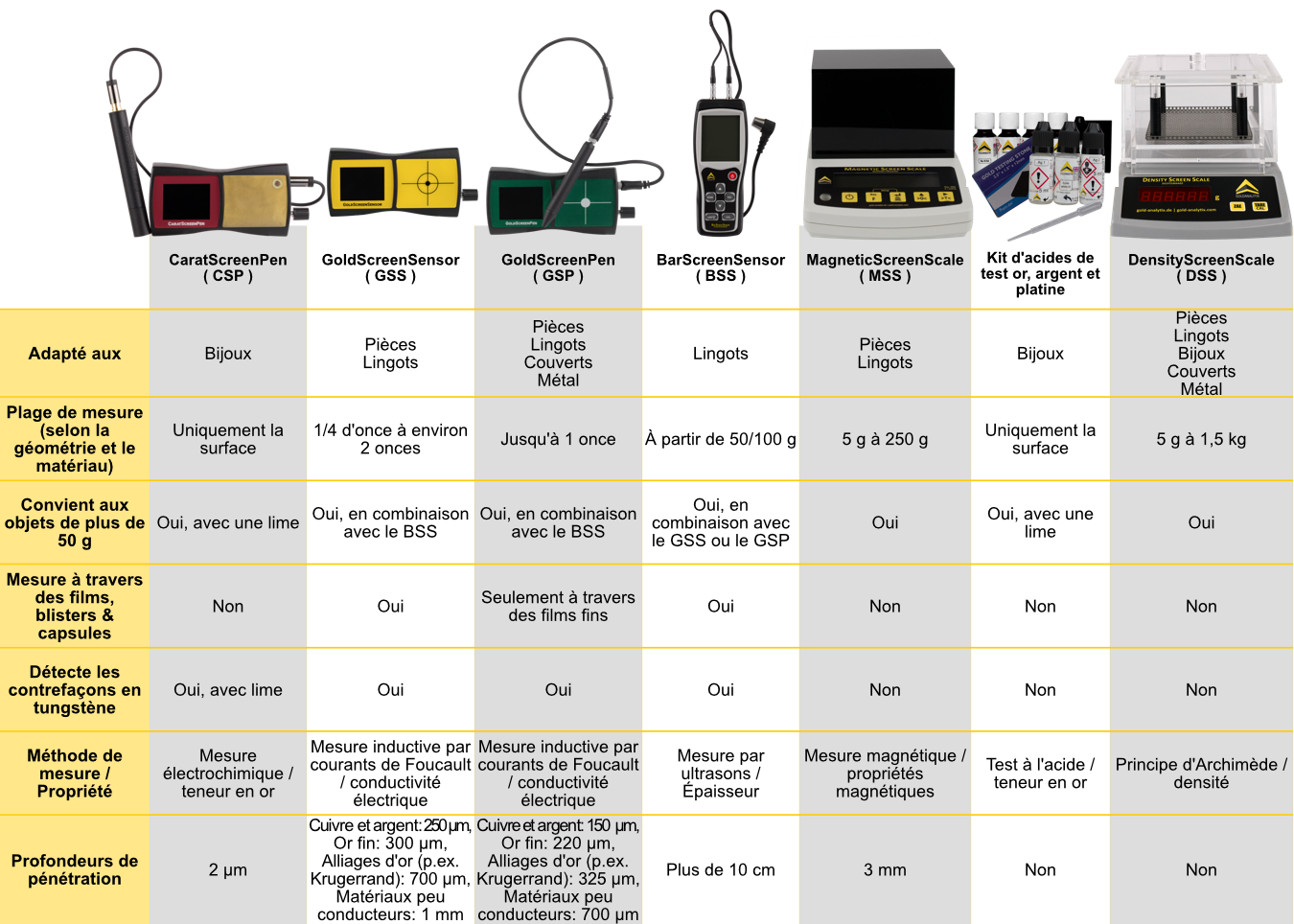
This depends entirely on which objects you want to check:
For classic investors (coins and bars), the GoldScreenSensor (also measures through packaging) in combination with a caliper and precision scale is usually sufficient . For bars from 50 to 100 grams upwards, we also recommend the BarScreenSensor, for which the objects must be unpacked. The GoldScreenPen can also be used for smaller investment objects up to approx. 1 ounce.
For gold jewellery, measurement with a combination of CaratScreenPen and DensityScreenScale is ideal. The fineness of gold (carat number) can also be determined with our test acid sets.
As a tungsten detector, the MagneticScreenScale is an alternative to the GoldScreenSensor for gold objects from approx. 1/4 ounce to 250 g. This MagneticScreenScale is a good choice for those who want to focus entirely on gold and do not want to unpack 100 gram bars, for example.
Goldanalytix manufactures and develops its own products to the highest quality standards in Regensburg, Germany, at the headquarters of its parent company MARAWE GmbH & Co. KG.
Anonymous purchase only possible below 2000 Euros:
If the purchase of gold, silver or other precious metals in Germany is to remain anonymous, the price of the purchased precious metal must not exceed a price of 1.999.99 Euros. For any sum exceeding this amount, the dealer is required by the Money Laundering Act to record personal details. Most countries have their own limitations, which should be researched before buying.
For large gold bars, the dealer surcharge is lower:
If a fixed amount is to be invested in physical precious metals, it is advisable to buy bars that are as large as possible, as the dealer's markup is comparatively large for small bars. A 1-ounce bar costs about € 1770 (as of August 2023). 1 ounce of gold in the form of 1 - gram bars, on the other hand, costs 1840 Euros. The price per gram exceeds the price of an ounce significantly. For coins, the premiums are also higher than for bars because of the minting.
Buy gold coins or 995 gold bars instead of paying VAT:
In Germany, if gold is to be purchased in bar form, it makes sense to buy pure gold bars (99.5% gold content), as no VAT is due for this purity or more. Gold coins are exempt from VAT from a purity of 90% on, but only if they were minted after 1800 and are or were considered official currency in their country of origin. In addition, the price may not exceed the material value of the gold content by more than 80% (some collector coins have an even higher market value). When buying silver or other precious metals from commercial dealers, the usual 19% German VAT is always charged in Germany.
Jewellery and collector coins are mainly suitable for connoisseurs:
With jewellery and collector coins made of precious metals (e.g. antique or rare coins), it should be noted that the value of the piece is not only composed of the pure metal price, but other factors can also influence the value. For jewellery, for example, the quality of workmanship, the manufacturer, or an additional gemstone can drive up the price. With coins, such an increase in price can be caused by the age, the country of origin or the depicted motif. A certain amount of expertise is required to determine value, and possible increases in the value of new coins are usually associated with speculative risks.
Small objects are usually easier to sell:
Even though the premium for smaller objects is higher, they are often easier to sell. You can both turn part of the investment amount back into cash (cutting up large bars reduces the sale value) and, in social crises, better use the small pieces as barter items.
Buy from the dealer or check for authenticity yourself with private sellers:
It is advisable to buy gold and other precious metals from reputable dealers. Otherwise, if one would like to save e.g. the value added tax (amounts to with precious metals except gold after all 19%) by private purchase, it should be examined whether the precious metal is genuine. The necessary tests can either be done by yourself (e.g. with the GoldScreenSensor) or by a precious metal dealer.
Purchase gold locally instead of buying a fake on the Internet:
It is not advisable to buy precious metals via private online trading platforms such as Ebay or Amazon, as fraudsters have an easy game there. It often happens that customers assume they have made a good deal and then only receive a fake made of copper with a thin layer of gold. However, if there is ever a genuine offer on these platforms, it is usually overpriced, as platforms such as Ebay or Amazon always demand fees from sellers. A personal sales meeting is preferable to sending a package when buying privately to be able to verify authenticity. Scammers would not allow you to do your own verification nor give you a return policy.
Hallmarking is not essential, yet it is not unimportant:
Hallmarking is standard on newer coins, jewellery or bars. In addition to the fineness, it sometimes provides further information about the precious metal, e.g. which manufacturer it comes from, what material it is made of or when the piece was manufactured.
Yes and no. Theoretically, it is possible to detect with a magnet whether an object has a dia- or paramagnetism. However, the magnetic forces of conventional magnets are often so low that they cannot be detected with the human eye. Therefore, a very strong magnet is needed for a measurement. We recommend the MagneticScreenScale that can measure down to hundredths of a gram. This enables the detection of very fine differences and the achievement of clear results.
We suggest that you repeat the measurement. Before a second measurement, please follow the steps below:
- Check that all components of the density measurement assembly are firmly seated on the scale and correctly arranged.
- If a completely unexpected result appears, restart the device and wait a few minutes.
- Carefully dry all components that should not be in contact with the water bath.
Now the scale should give you a valid result.
In addition, it may be that there are cavities in the material that are not visible from the outside or that the object is more porous on the inside than it appears from the outside. Also not expected contamination with denser or less dense materials/substances can be responsible for a wrong answer.
If you have any further questions, please do not hesitate to contact us.
This depends entirely on which objects you want to check:
For classic investors (coins and bars), the GoldScreenSensor (also measures through packaging) in combination with a caliper and precision scale is usually sufficient . For bars from 50 to 100 grams upwards, we also recommend the BarScreenSensor, for which the objects must be unpacked. The GoldScreenPen can also be used for smaller investment objects up to approx. 1 ounce.
For gold jewellery, measurement with a combination of CaratScreenPen and DensityScreenScale is ideal. The fineness of gold (carat number) can also be determined with our test acid sets.
As a tungsten detector, the MagneticScreenScale is an alternative to the GoldScreenSensor for gold objects from approx. 1/4 ounce to 250 g. This MagneticScreenScale is a good choice for those who want to focus entirely on gold and do not want to unpack 100 gram bars, for example.
When measuring with the BarScreenSensor, it is important to remove the packaging of the object and apply the ultrasound gel to the measuring head. The measuring head must be in direct contact with the test piece.
It is also necessary to scan the entire ingot. It is possible that foreign metal inclusions can only be found at certain points within the object. In addition, the ingot should be physically measured beforehand so that the measured ultrasonic value can be compared with the real thickness.
Furthermore, it is important to pay attention to the size/thickness of the object. If it is too thin, the BarScreenSensor could give wrong results. Therefore, please use the BarScreenSensor only for bars of 50 to 100 grams or more. In addition, you should use at least one other measuring method (e.g. digital caliper and precision scale).
The smaller the bar to be tested, the more embossing and stamps in the gold/silver have an effect on the measurement result.
- With experience it takes about 30 seconds to take a measurement and calculate the result.
- Make sure that the scale is located as steady and vibration-proof as possible.
- Ideally, start the device 5-10 minutes before you use it so that it can regulate itself.
- The density scale works most reliably in a stable environment with a room temperature of 20-25 °C. The most important thing is that the temperature remains constant during the time of operation or use.
- Also note that you cannot use the density scale to measure jewellery set with precious stones, as stones falsify the density. Also, do not weigh any substances that react with water, e.g. sodium or potassium, or have a water-soluble layer.
- The test specimen must be dry and clean.
- Remove any capsules and blisters before measuring, otherwise the result will be inconclusive.
- With weakly wetting liquids (e.g. normal water), air bubbles may accumulate on the carrier or the test piece. An air bubble with a diameter of 2 mm already causes a measurement inaccuracy of 4 mg.
The density scale only indicates the weight of the test piece in the water. To calculate the density, you need the normal weight of the piece and the weight of the object in the water. To free you from having to do your own calculations, we provide you with our Excel calculation tool, which does the maths for you. Simply enter your measured values and the density of your piece is calculated.
Basically, density can be calculated by dividing the mass of an object by its volume.
If you have the DensityScreenScale or the bar testing set GAX1000, then you can use our calculation tool for this. To do this, measure the weight in air and the weight in water with the density scale. You can enter these two values into the tool and it will give you the density. In addition, with the calculation tool you have comparative values of some alloys and many pure metals.
If you want to calculate the density yourself, the formula is:
Density [g/cm³] = Air value [g] / (Air value [g] - Water value [g])
The measuring range of the density scale goes from 0.01 - 2000 grams. This means you can measure small objects as well as bars up to a weight of 2 kg.
Caution: With silver bars over 1 kilogram, the measuring basket may not be large enough, as silver has a lower density than gold and is therefore larger for the same weight.
This depends entirely on which objects you want to check:
For classic investors (coins and bars), the GoldScreenSensor (also measures through packaging) in combination with a caliper and precision scale is usually sufficient . For bars from 50 to 100 grams upwards, we also recommend the BarScreenSensor, for which the objects must be unpacked. The GoldScreenPen can also be used for smaller investment objects up to approx. 1 ounce.
For gold jewellery, measurement with a combination of CaratScreenPen and DensityScreenScale is ideal. The fineness of gold (carat number) can also be determined with our test acid sets.
As a tungsten detector, the MagneticScreenScale is an alternative to the GoldScreenSensor for gold objects from approx. 1/4 ounce to 250 g. This MagneticScreenScale is a good choice for those who want to focus entirely on gold and do not want to unpack 100 gram bars, for example.
When measuring with the BarScreenSensor, it is important to remove the packaging of the object and apply the ultrasound gel to the measuring head. The measuring head must be in direct contact with the test piece.
It is also necessary to scan the entire ingot. It is possible that foreign metal inclusions can only be found at certain points within the object. In addition, the ingot should be physically measured beforehand so that the measured ultrasonic value can be compared with the real thickness.
Furthermore, it is important to pay attention to the size/thickness of the object. If it is too thin, the BarScreenSensor could give wrong results. Therefore, please use the BarScreenSensor only for bars of 50 to 100 grams or more. In addition, you should use at least one other measuring method (e.g. digital caliper and precision scale).
The smaller the bar to be tested, the more embossing and stamps in the gold/silver have an effect on the measurement result.
No, we recommend the BarScreenSensor only from a weight of 50 to 100 grams and coins do not normally reach this value. If the test objects are too thin, the path length of the ultrasound is not long enough to perform a sufficiently precise measurement.
The GoldScreenSensor which can test smaller objects like coins and bars up to approx. 2 ounces, is an excellent addition to the BarScreenSensor.
No. For ultrasound measurement with the BarScreenSensor, the measuring head combined with an ultrasound gel (included in the delivery) must be in direct contact with the smooth surface of the object.
To measure through thin packaging and blisters, devices such as the GoldScreenSensor are more suitable.
No, unfortunately that is not possible with these products.
The zinc object must be clean and free of residues such as dirt, oil or particles. The object is then degreased, e.g. with the TIFOO Galvano Degreaser. Depending on the size, the object is immersed in the yellow chromate for a few minutes. 3 minutes is a good guideline.
In principle, it is possible to gold-plate some aluminium alloys with the Gold Star, but so far this only works with a few compositions. The success of the application can therefore not be guaranteed for all aluminium alloys.
Our products for electroless silver plating only deposit on copper and brass as a base, as the products can only deposit silver with these materials. Accordingly, the products for electroless silver plating can NOT be deposited on silver. If copper or brass are coated, a thin silver layer of 200 - 300 nm is produced, which is intended for decorative purposes only. If you want a thicker silver layer on your object, the only process left is electroplating!
Yes, this is possible with the help of our conductive copper varnish. The object is sprayed or brushed with copper conductive paint or copper conductive lacquer spray, which first creates a metallic layer. The object is then given its final conductivity in a galvanic bath with acidic bright copper electrolyte. This creates a continuous, conductive copper layer that can then be further treated like any other copper layer, e.g. by silver plating.
Rust Piranha can normally be painted over or treated further. However, please check on an inconspicuous spot whether the rust converter is compatible with your paint colour.
This depends entirely on which objects you want to check:
For classic investors (coins and bars), the GoldScreenSensor (also measures through packaging) in combination with a caliper and precision scale is usually sufficient . For bars from 50 to 100 grams upwards, we also recommend the BarScreenSensor, for which the objects must be unpacked. The GoldScreenPen can also be used for smaller investment objects up to approx. 1 ounce.
For gold jewellery, measurement with a combination of CaratScreenPen and DensityScreenScale is ideal. The fineness of gold (carat number) can also be determined with our test acid sets.
As a tungsten detector, the MagneticScreenScale is an alternative to the GoldScreenSensor for gold objects from approx. 1/4 ounce to 250 g. This MagneticScreenScale is a good choice for those who want to focus entirely on gold and do not want to unpack 100 gram bars, for example.
- With experience it takes about 30 seconds to take a measurement and calculate the result.
- Make sure that the scale is located as steady and vibration-proof as possible.
- Ideally, start the device 5-10 minutes before you use it so that it can regulate itself.
- The density scale works most reliably in a stable environment with a room temperature of 20-25 °C. The most important thing is that the temperature remains constant during the time of operation or use.
- Also note that you cannot use the density scale to measure jewellery set with precious stones, as stones falsify the density. Also, do not weigh any substances that react with water, e.g. sodium or potassium, or have a water-soluble layer.
- The test specimen must be dry and clean.
- Remove any capsules and blisters before measuring, otherwise the result will be inconclusive.
- With weakly wetting liquids (e.g. normal water), air bubbles may accumulate on the carrier or the test piece. An air bubble with a diameter of 2 mm already causes a measurement inaccuracy of 4 mg.
The measuring range of the density scale goes from 0.01 - 2000 grams. This means you can measure small objects as well as bars up to a weight of 2 kg.
Caution: With silver bars over 1 kilogram, the measuring basket may not be large enough, as silver has a lower density than gold and is therefore larger for the same weight.
The density scale only indicates the weight of the test piece in the water. To calculate the density, you need the normal weight of the piece and the weight of the object in the water. To free you from having to do your own calculations, we provide you with our Excel calculation tool, which does the maths for you. Simply enter your measured values and the density of your piece is calculated.
Basically, density can be calculated by dividing the mass of an object by its volume.
If you have the DensityScreenScale or the bar testing set GAX1000, then you can use our calculation tool for this. To do this, measure the weight in air and the weight in water with the density scale. You can enter these two values into the tool and it will give you the density. In addition, with the calculation tool you have comparative values of some alloys and many pure metals.
If you want to calculate the density yourself, the formula is:
Density [g/cm³] = Air value [g] / (Air value [g] - Water value [g])
Yes, gold has a density of 19.32 g/cm³, but the popular counterfeit material wolfram has a density of 19.30 g/cm³. For silver, which has a density of 10.49 g/cm³, lead-tin or molybdenum alloys with a similar density are often used. Thus, a counterfeit cannot be ruled out with a density measurement alone.
This depends entirely on which objects you want to check:
For classic investors (coins and bars), the GoldScreenSensor (also measures through packaging) in combination with a caliper and precision scale is usually sufficient . For bars from 50 to 100 grams upwards, we also recommend the BarScreenSensor, for which the objects must be unpacked. The GoldScreenPen can also be used for smaller investment objects up to approx. 1 ounce.
For gold jewellery, measurement with a combination of CaratScreenPen and DensityScreenScale is ideal. The fineness of gold (carat number) can also be determined with our test acid sets.
As a tungsten detector, the MagneticScreenScale is an alternative to the GoldScreenSensor for gold objects from approx. 1/4 ounce to 250 g. This MagneticScreenScale is a good choice for those who want to focus entirely on gold and do not want to unpack 100 gram bars, for example.
For jewellery, there are many different alloys with a wide variety of metals. Since the magnetic scale measures very finely, even a small proportion of a ferro- or paramagnetic metal (e.g. nickel, platinum) can influence the result, making it no longer meaningful. Therefore, the magnetic balance is not suited for jewellery testing. The right choice in this case would be our CaratScreenPen
The magnet of the MagneticScreenScale is a strong neodymium magnet that must be handled with care. On the one hand, electronic devices and objects with magnetic storage (hard disks, credit cards) should be kept away from the magnet, otherwise they can be damaged. Secondly, you should keep it away from other strong magnets to avoid bruising, splintering or sparks.
People with pacemakers should also keep a sufficient distance from this magnet.
Information sheet: "Safe handling of magnets"
- Be careful when handling the magnet, otherwise it may cause injury.
- There should be no electronic devices in the vicinity, as these can be damaged by the neodymium magnet.
- An electrostatic charge can falsify the measurement result, so make sure to discharge the packaging or the plexiglass structure with the anti-static spray.
- Test the objects for ferromagnetic materials/impurities beforehand with the supplied bar magnet, otherwise the scale may be damaged or the result is incorrect.
If you get a positive result from the magnetic balance, then the material at hand is diamagnetic. If the result is weakly negative, then it is paramagnetic and if it is strongly negative, then the object is probably ferromagnetic. In most cases, a negative result means that the test piece is not a pure precious metal. However, there are some exceptions to consider: e.g. older Krugerrand coins.
No, it is not possible to make any statements about precious metal alloys with the magnetic balance, as the differences are far too small. It is only possible to determine whether the test specimens are made of a counterfeit material.